Cảbon, a fundamental element abundantly found in nature, holds immense significance in various fields of science and technology. From its role as the building block of life to its versatile applications in industries such as energy, materials, and electronics, Cảbon has revolutionized the way we understand and interact with the world. In this article, we delve into the fascinating world of Cảbon, exploring its properties, applications, and the profound impact it has on our daily lives. Carbon is an element that plays an indispensable role in the tapestry of life on Earth. It’s the essence of diamonds and graphite, the backbone of organic compounds, and the key to sustaining all living organisms. But what is carbon?
Carbon is a chemical element with the symbol C and atomic number 6. It is nonmetallic and tetravalent, meaning it has four valence electrons. Carbon is the second most abundant element in the universe, after hydrogen.
Carbon is found in all living things and is essential for life. It is a component of all organic compounds, including proteins, carbohydrates, and lipids. Carbon is also found in many inorganic compounds, such as limestone and coal.
Carbon has a wide range of properties that make it unique. It is a strong and lightweight material, making it ideal for use in construction and manufacturing. Carbon is also a good conductor of electricity, making it useful in electronics.
Carbon is used in a wide variety of products, including:
- Plastics
- Fibers
- Fuels
- Pharmaceuticals
- Food
- Cosmetics
- Construction materials
- Electronics
Carbon is also essential for the environment. It plays a role in the global carbon cycle, which helps to regulate the Earth’s climate. Carbon is also a component of soil organic matter, which is essential for plant growth.
Carbon is a genuinely elemental wonder. It is essential for life, versatile, and abundant. Carbon plays a vital role in our world, and we are only beginning to understand its full potential.
What is Cảbon?
Carbon is a versatile and ubiquitous element, represented by the symbol “C” on the periodic table. It’s the fourth-most abundant element in the universe by mass, and its significance extends far beyond the realm of astrophysics. In the human body, it holds the distinction of being the second-most prevalent element, surpassed only by oxygen. Carbon’s importance stems from its extraordinary ability to form a diverse array of compounds, a quality driven by its possession of four valence electrons.
These valence electrons grant carbon its unique bonding capabilities, allowing it to create intricate and varied chemical structures. This remarkable property makes carbon the cornerstone of organic chemistry, where it serves as the fundamental building block for life itself. Whether as the backbone of complex biological molecules, the essence of precious gemstones, or the key ingredient in a multitude of materials, carbon’s adaptability and versatility are unrivaled. In the exploration that follows, we will uncover the captivating story of carbon, from its role in the chemistry of life to its myriad applications across diverse fields of science and technology.
Cảbon: The Basis of Life
Cảbon in Biological Structures
Carbon is the backbone of organic molecules, making it essential for life on Earth. We examine its role in biological structures, from DNA and proteins to carbohydrates and lipids.
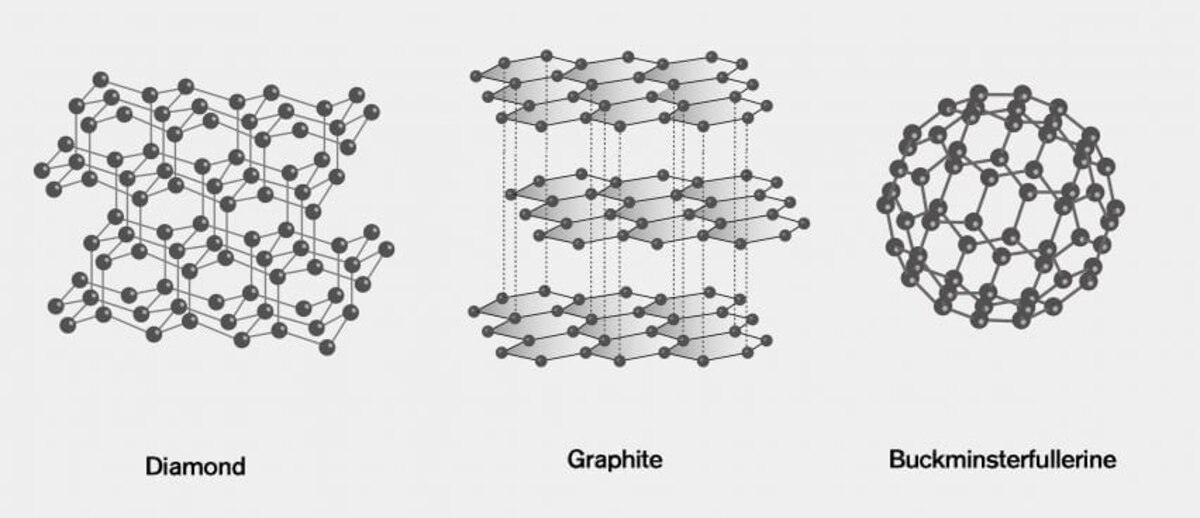
Carbon Allotropes: A World of Possibilities
From Diamonds to Graphite
Carbon exists in various allotropes, each with unique properties. We explore the differences between diamonds, graphite, and other carbon forms, shedding light on their applications.
Ref Link- https://www.dezeen.com/2021/06/25/carbon-allotropes-buckminsterfullerene-andrei-khlobystov/
Carbon-Based Materials and Applications
Carbon Fiber:
Carbon fiber composites are lightweight yet solid and durable materials widely used in aerospace, automotive, and sports industries. Their high strength-to-weight ratio makes them ideal for applications that require strength and rigidity, such as aircraft components and high-performance sporting equipment.
Graphene:
Graphene, a single layer of carbon atoms arranged in a two-dimensional lattice, possesses exceptional electrical and thermal conductivity, along with remarkable mechanical properties. It holds immense potential for advancements in electronics, energy storage, and sensor technologies, paving the way for innovations like flexible displays, high-speed transistors, and efficient energy storage devices.
Carbon Nanotubes:
Carbon nanotubes are cylindrical structures composed of rolled-up graphene sheets. They exhibit extraordinary strength, thermal conductivity, and electrical properties. Carbon nanotubes find applications in areas such as nanoelectronics, nanomedicine, and materials reinforcement, opening up possibilities for ultra-small electronic devices, targeted drug delivery systems, and more robust composite materials.
The carbon atom, the unassuming hero of the periodic table, is a fundamental building block of matter. It plays an essential role in the chemistry of life and the universe. Let’s embark on a journey to explore the intricacies of this tiny yet remarkable entity.
Atomic Structure:
At the heart of a carbon atom lies a minor yet dense nucleus. This nucleus is composed of protons, positively charged subatomic particles, and neutrons, electrically neutral particles. Orbiting around the nucleus are electrons, negatively charged particles. Carbon, the sixth element on the periodic table, boasts six electrons in its atomic makeup. These electrons are arranged within the atom’s energy levels or orbitals.
Electron Configuration:
Within the electron cloud that envelops the nucleus, the first energy level accommodates two electrons, while the second level houses four electrons. Carbon’s electron configuration is expressed as 1s² 2s² 2p², indicating the distribution of its six electrons.
Valence Electrons:
Among these electrons, the outermost energy level, the second level, harbors four of them. These are known as valence electrons, and they play a pivotal role in chemical bonding. Carbon’s possession of four valence electrons endows it with the capacity to form stable compounds through covalent bonding.
Covalent Bonding:
One of the key features of carbon is its ability to engage in strong covalent bonds. It readily shares electrons with other atoms, particularly fellow carbon atoms, as well as elements like hydrogen, oxygen, nitrogen, and more. This aptitude for forming bonds with other features in various ways results in the creation of an astonishing diversity of molecules.
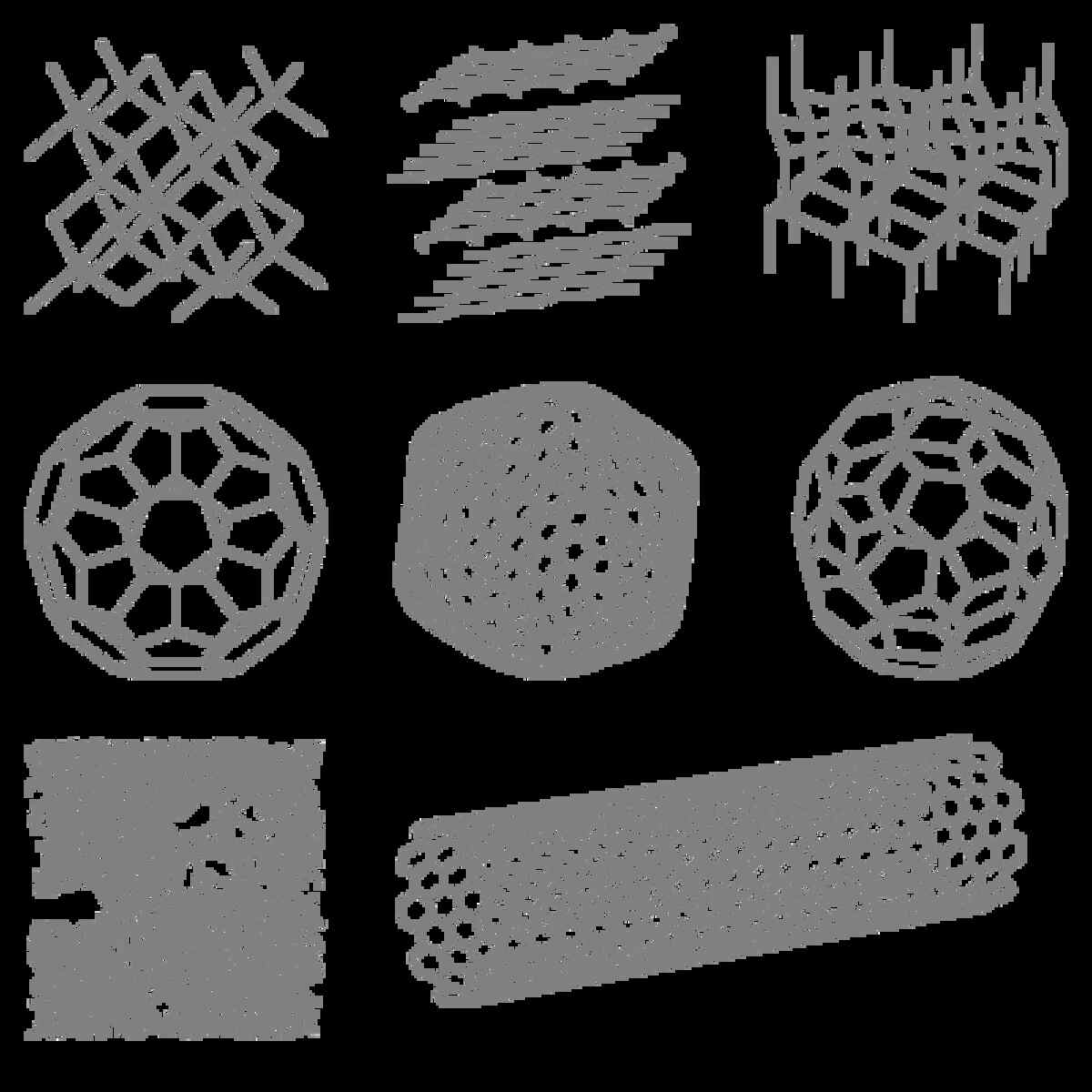
Ref Link- https://www.researchgate.net/figure/Structures-of-selected-allotropes-of-carbon_fig1_288835294
Allotropes:
Carbon showcases different structural forms known as allotropes. Two well-known carbon allotropes are graphite and diamond. In graphite, carbon atoms arrange themselves in layers, held together by relatively weak forces. On the other hand, diamond exhibits a three-dimensional lattice structure, where each carbon atom forms strong bonds with four other carbon atoms, producing a solid and rigid material.
Life’s Backbone:
Carbon serves as the backbone of organic molecules, laying the foundation for life on Earth. Organic compounds, including carbohydrates, proteins, lipids, and nucleic acids, all revolve around the central role of carbon atoms. The versatility of carbon’s bonding capabilities allows for the complex and diverse structures that define living organisms.
Carbon’s Cosmic Role:
Beyond Earth, carbon plays a significant role in the cosmos. It is abundant on our planet and extends its influence to the far reaches of the universe. Carbon is formed within the cores of massive stars through intricate nuclear fusion processes. During supernova explosions, carbon and other elements are ejected into space, eventually leading to the creation of new stars, planets, and life-sustaining environments.
The carbon atom, with its unique electron configuration and unparalleled bonding capabilities, is the cornerstone of chemistry and life as we know it. Its influence spans from the tiniest particles within the atom to the vast expanse of the universe, making it a central character in the story of the cosmos and the chemistry of life.
Carbon’s Impact on Energy and Sustainability
Fossil Fuels and Carbon Emissions:
Carbon-based energy sources, such as coal, oil, and natural gas, have been integral to the development of modern society. However, the combustion of these fossil fuels releases carbon dioxide (CO2), contributing to climate change. The increasing focus on sustainable energy has led to advancements in carbon capture and storage technologies, aiming to reduce CO2 emissions and mitigate their impact on the environment.
Renewable Energy and Carbon Materials:
Furthermore, carbon-based materials play a crucial role in renewable energy technologies. For example, carbon electrodes are used in fuel cells, while carbon-based materials are vital components of photovoltaic cells and energy storage devices like lithium-ion batteries, enabling the transition to a cleaner and more sustainable energy future.
The Many Faces of Carbon
Carbon is a versatile element with myriad faces, and its applications are as diverse as its forms.
Carbon in Industry: Fueling Progress
One of the most prevalent uses of carbon is as a source of energy. Fossil fuels, such as coal, oil, and natural gas, are rich in carbon compounds. When burned, they release energy that powers our homes, vehicles, and industries. However, the combustion of fossil fuels also releases carbon dioxide into the atmosphere, contributing to climate change.
Efforts are underway to develop cleaner and more sustainable energy sources, such as solar and wind power, to reduce our reliance on carbon-intensive fuels.
Carbon’s unique properties have also found applications in technology. Carbon fiber, for instance, is renowned for its exceptional strength-to-weight ratio, making it ideal for aerospace and sports equipment use. On the other hand, carbon nanotubes are nanoscale cylinders of carbon atoms with remarkable electrical and thermal conductivity, holding promise for future technologies.
Carbon in Medicine: Healing and Diagnosis
In the field of medicine, carbon compounds play pivotal roles. Carbon-14, a radioactive isotope of carbon, is used in radiocarbon dating to determine the age of archaeological artifacts and fossils. Carbon-based molecules are also essential in pharmaceuticals, serving as the building blocks for many drugs.

Ref Link- https://www.dezeen.com/2021/06/25/carbon-allotropes-buckminsterfullerene-andrei-khlobystov/
Carbon in Nature: Ecological Balance
Carbon is not only found in human-made compounds but also in natural substances. It is a fundamental element in the structure of plants and animals. Carbon plays a vital role in forming calcium carbonate shells and skeletons by ocean marine organisms like corals and mollusks.
Carbon Footprint: Environmental Impact
While carbon is essential for life, its overabundance in the atmosphere, primarily in the form of carbon dioxide (CO2), has raised concerns about its impact on climate change. Excessive CO2 emissions from human activities, such as burning fossil fuels and deforestation, have led to a rise in global temperatures and destabilized ecosystems.
Efforts to reduce our carbon footprint, such as transitioning to renewable energy sources and implementing reforestation projects, are crucial in mitigating these effects. Carbon’s presence in various facets of life, from energy to technology, medicine, and nature, and its environmental impact highlights the necessity of responsible and sustainable carbon management.
Conclusion
Carbon, the versatile element at the heart of life and technology, continues to shape our world in countless ways. From its role in the chemistry of life to its applications in materials science, electronics, and energy, carbon’s remarkable properties and adaptability have revolutionized various industries. As we strive for a sustainable and technologically advanced future, understanding and harnessing the potential of carbon will undoubtedly play a crucial role in shaping our progress.

